the change in concentration of a reacant that is a first order reaction?
This graph should be straight for a second order reaction.
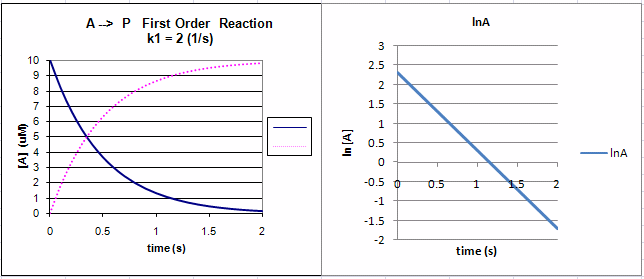
The half life of a first order reaction is independent of concentration.
For a first order reaction A Arrow

This graph shows a comparison between the rate of reaction between the

Chemical reaction of known as athe reaction i aq bg, has Rate

(c) The following mixed second order rate constants for this reaction have

equation 3), as well as the rate of reaction of injected electrons with

The catalyst speeds up the rate of reaction without being consumed;

The variation in the rate of reaction catalysed by porous spherical

The reaction rates are as follows:
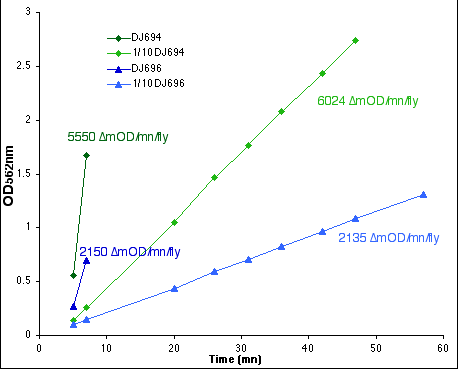
and thus the rate of reaction only depends on enzyme concentration.
2nd Order Reaction

Figure 3: Rate constant for the reaction of silicon atoms with O2 as a

each model: the top line represents the reaction rate combination: UL LL
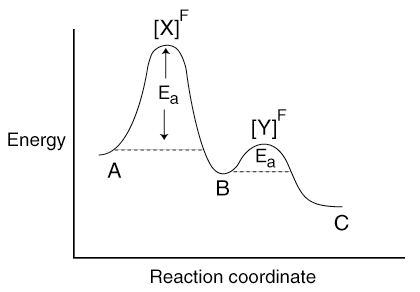
Here's a hypothetical plot of reaction coordinate vs. energy of the reaction

The reaction order of reaction (10) is 1 towards NO2.

Arroyo orders quick reaction teams, mobile testing for A(H1N1). Jun 27, 2009 | 11:34 PM. MANILA, Philippines - President Gloria Macapagal-Arroyo on Saturday
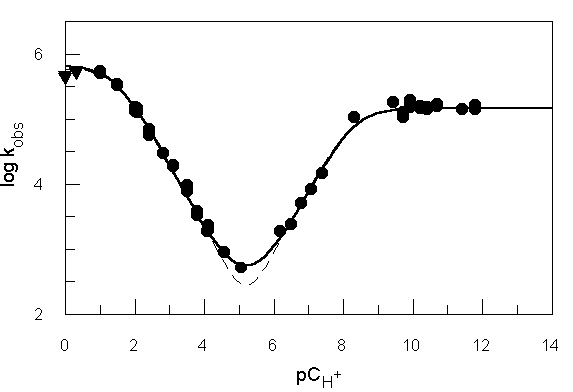
in determining reaction rates. This provides a method of calculating

secondary structre to the reaction rate could be determined,

No comments:
Post a Comment